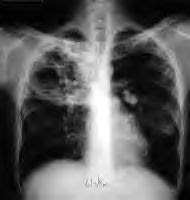
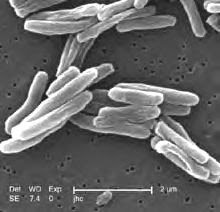
A new tuberculosis drug given special status by both U.S. and European regulators might lead to simpler, more effective TB treatment regimens.Approximately one-third of the world’s population is infected with the tuberculosis bacterium, and approximately 1.6 million people died of the disease during 2005.Currently, TB patients must adhere to a complex treatment regimen over a six- to nine-month period. That demanding schedule, researchers said, often results in patients skipping treatment doses, which, in turn, gives rise to drug-resistant strains such as multi-drug-resistant, or MDR, tuberculosis and the recently identified extensively drug-resistant form of the disease.
The new drug, SQ109, is an antimicrobial agent developed through a partnership between the National Institute of Allergy and Infectious Diseases and the biotech company Sequella Inc.SQ109 has recently been granted "orphan drug" status by the U.S. Food and Drug Administration and the European Medicines Agency. The orphan designation involves tax reductions and marketing exclusivity to encourage development of drugs to treat diseases affecting fewer than 200,000 people.
http://tinyurl.com/yp3u6o
WHAT'S NEW IN TUBERCULOSIS
Sunday, 11 November 2007
Subscribe to:
Post Comments (Atom)



No comments:
Post a Comment