http://www.bizjournals.com/seattle/stories/2008/08/25/daily19.html?ana=from_rss
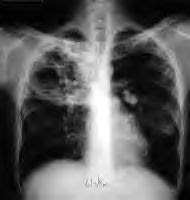
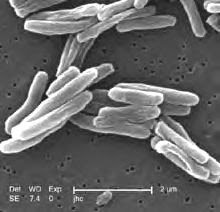
The Infectious Disease Research Institute (IDRI) said it’s been awarded a $6.3 million federal grant to study a new tuberculosis vaccine.The four-year grant is from the National Institute of Allergy and Infectious Diseases, part of the National Institutes of Health. Tuberculosis vaccines haven’t changed much in many years, according to researchers at the Seattle nonprofit.“The currently available vaccine, Bacillus Calmette-Guerin (BCG), was developed in 1921 and fails to protect most people beyond childhood,” said Rhea Coler of IDRI, said in a statement.IDRI is focusing on the making of adjuvants — an essential ingredient in several vaccines — that it believes can target specific immune pathways and improve vaccine protection
WHAT'S NEW IN TUBERCULOSIS
Thursday, 28 August 2008
Wednesday, 27 August 2008
TB in SIBERIA
TOMSK, RUSSIA—Prisoners in western Siberia who contract tuberculosis (TB) get sent to a forbidding complex in the heart of this provincial city. Armed guards with dogs patrol the nearby streets. Barbed wire covers the top of the outer walls. Iron bars clang shut when anyone enters. TB can keep you out of a remote Siberian prison camp, but it doesn't keep you out of jail.
And a decade ago, passing through this prison hospital's portals also posed a significant risk of premature death. Between 1991 and 2001 the incidence of TB in Russia's prisons reached a staggering 7,000 cases per 100,000 inmates, according to one estimate. Prisoners made up 25 percent of all new cases in the nation. In this oil-rich province the size of New Mexico with just over a million inhabitants, the prison TB rate reached the equivalent of 4,000 cases per 100,000 inmates, with nearly one of every 11 cases proving fatal.
The massive economic dislocation that accompanied the collapse of the Soviet Union turned Russia into an ideal breeding ground for a TB epidemic. Unemployment and alcoholism skyrocketed. Health and social services collapsed. As petty theft and violent crime soared, the prison population swelled to more than a million, with millions more moving in and out of incarceration. Many developed TB either because their immune systems, weakened by drugs, alcohol and poor nutrition, could no longer keep latent TB in check (an estimated one third of the world's population has latent TB) or they caught it from other prisoners.
The prisons in turn became an "epidemiological pump" for spreading the disease throughout the general population. Ex-prisoners, often with improperly treated TB that had mutated into the multidrug resistant form of the disease, moved back into cramped apartment blocs where, during the long, cold Siberian winters, hallways and unventilated apartments provided ideal conditions for airborne transmission to unwary neighbors, friends and family members। The annual rate of new TB cases among the general population in Russia more than doubled in the 1990s to 88 cases per 100,000 inhabitants. In Siberia the rate soared to over 130 new cases per 100,000 souls. For a comparison, the U.S. had around 10 cases per 100,000 residents per year during the same period, and currently has about four cases per 100,000 annually.
http://www.sciam.com/article.cfm?id=prison-plague-post-soviet-russia
And a decade ago, passing through this prison hospital's portals also posed a significant risk of premature death. Between 1991 and 2001 the incidence of TB in Russia's prisons reached a staggering 7,000 cases per 100,000 inmates, according to one estimate. Prisoners made up 25 percent of all new cases in the nation. In this oil-rich province the size of New Mexico with just over a million inhabitants, the prison TB rate reached the equivalent of 4,000 cases per 100,000 inmates, with nearly one of every 11 cases proving fatal.
The massive economic dislocation that accompanied the collapse of the Soviet Union turned Russia into an ideal breeding ground for a TB epidemic. Unemployment and alcoholism skyrocketed. Health and social services collapsed. As petty theft and violent crime soared, the prison population swelled to more than a million, with millions more moving in and out of incarceration. Many developed TB either because their immune systems, weakened by drugs, alcohol and poor nutrition, could no longer keep latent TB in check (an estimated one third of the world's population has latent TB) or they caught it from other prisoners.
The prisons in turn became an "epidemiological pump" for spreading the disease throughout the general population. Ex-prisoners, often with improperly treated TB that had mutated into the multidrug resistant form of the disease, moved back into cramped apartment blocs where, during the long, cold Siberian winters, hallways and unventilated apartments provided ideal conditions for airborne transmission to unwary neighbors, friends and family members। The annual rate of new TB cases among the general population in Russia more than doubled in the 1990s to 88 cases per 100,000 inhabitants. In Siberia the rate soared to over 130 new cases per 100,000 souls. For a comparison, the U.S. had around 10 cases per 100,000 residents per year during the same period, and currently has about four cases per 100,000 annually.
http://www.sciam.com/article.cfm?id=prison-plague-post-soviet-russia
Friday, 22 August 2008
OPTIONS FOR SCREENING AND TREATMENT OF TUBERCULOSIS REVIEWED
Screening for M tuberculosis infection should be targeted to persons at high risk for infection or progression to active disease. Risk factors for tuberculosis include birth in an endemic country, economically disadvantaged status, and immunosuppressive conditions. Although the TST is the standard test for diagnosis of M tuberculosis, antigen-specific interferon-gamma release assays are useful, particularly in persons with previous bacille Calmette-Guérin vaccination or possible nontuberculous mycobacteria.
The treatment of choice for most patients with latent tuberculosis infection is isoniazid monotherapy, except for those in whom primary drug-resistant tuberculosis is suspected. Directly observed combination therapy with isoniazid, rifampin, pyrazinamide, and ethambutol should be promptly started once active tuberculosis is diagnosed. Combination therapy should be administered for a 2-month "intensive phase" and should, in most cases, be followed by treatment with isoniazid and a rifamycin product for a 4- to 7-month "continuation phase."
http://www.medscape.com/viewarticle/579389
The treatment of choice for most patients with latent tuberculosis infection is isoniazid monotherapy, except for those in whom primary drug-resistant tuberculosis is suspected. Directly observed combination therapy with isoniazid, rifampin, pyrazinamide, and ethambutol should be promptly started once active tuberculosis is diagnosed. Combination therapy should be administered for a 2-month "intensive phase" and should, in most cases, be followed by treatment with isoniazid and a rifamycin product for a 4- to 7-month "continuation phase."
http://www.medscape.com/viewarticle/579389
Thursday, 21 August 2008
ACTION OF RIFAMYCIN ANTIBIOTICS
http://newswire.rockefeller.edu/?page=engine&id=799
Rifamycin antibiotics attack tuberculosis bacteria with walls, not signals
Amid concerns about the rising number of new tuberculosis cases worldwide, researchers led by Rockefeller University’s Seth A. Darst have reexamined and disproved a theory that describes how a potent class of antibiotics kills a deadly form of bacteria. The findings, which will appear in this week’s online issue of the Proceedings of the National Academy of Sciences, not only bring scientists closer to understanding how these antibiotics work but also how the bacteria become resistant to their effects.The class of antibiotics, called rifamycins, was developed in the 1950s to combat tuberculosis-causing bacteria. The problem, however, was that the bacteria fought back, quickly developing resistance. And the rate of decline for new tuberculosis cases has begun to slow during the past decade, with more than nine million people across the globe currently afflicted.
Rifamycins kill their prey by binding to RNA polymerase, the enzyme that kicks off gene expression by transcribing DNA to messenger RNA. However, the exact mechanism by which rifamycins interfere with the process had long remained unknown. A breakthrough came in 2001, when Elizabeth Campbell, a research associate in Darst’s Laboratory of Molecular Biophysics, and her colleagues showed that rifamycins bind next to RNA polymerase’s active center such that the rifamycin acts like a wall, physically blocking RNA from elongating. These results supported a steric-occlusion model for rifamycin action that explained — and continues to explain — past findings.But the newer model, proposed three years ago, describes a very different mechanism. Called the allosteric model, it proposes that rifamycins do, indeed, bind to the enzyme next to the enzyme’s active center, but instead of blocking the elongating RNA molecule, rifamycins transmit a signal to the enzyme’s active center, decreasing a magnesium ion’s ability to bind. Without the magnesium ion, Mg2+, RNA cannot be transcribed.“It was a beautiful model, but there were parts of it that didn’t add up and those parts directly conflicted with the model published in 2001,” says lead researcher Andrey Feklistov, a postdoc in the Darst lab who conducted the research along with several colleagues at Rockefeller, the Waksman Institute of Microbiology at Rutger’s University and The Public Health Research Institute of New Jersey Medical School.The steric-occlusion model suggested that the stronger the rifamycin binds — that is, the sturdier the wall — the better it would work to halt transcription. But according to the allosteric model proposed by a team from The Ohio State University, that wasn’t necessarily the case. Even if rifamycins bind strongly, the enzyme could still be rifamycin-resistant due to a blip along the long signaling pathway. “So we did what scientists do,” says Feklistov. “We took another look.”By testing the same two mutant strains of RNA polymerase that the Ohio team used, ones that had mutations along the proposed signaling pathway, the Darst team found that the mutants were resistant to rifamycin precisely because the antibiotic could not bind tightly to the enzyme. “This suggests that the steric-occlusion model best explains the available biochemical and structural evidence that has been published,” says Feklistov. Moreover, the Darst team found that rifamycins have no effect on metal ion binding to the active center, in direct contradiction to the allosteric model.Understanding the mechanism by which rifamycins kill bacteria allows scientists to better understand how the tuberculosis-causing bacteria develop resistance to the antibiotics — and develop drugs to combat this effect. “At this stage,” says Feklistov, “any evidence, positive or negative, will help focus our attention toward this goal.”
Rifamycin antibiotics attack tuberculosis bacteria with walls, not signals
Amid concerns about the rising number of new tuberculosis cases worldwide, researchers led by Rockefeller University’s Seth A. Darst have reexamined and disproved a theory that describes how a potent class of antibiotics kills a deadly form of bacteria. The findings, which will appear in this week’s online issue of the Proceedings of the National Academy of Sciences, not only bring scientists closer to understanding how these antibiotics work but also how the bacteria become resistant to their effects.The class of antibiotics, called rifamycins, was developed in the 1950s to combat tuberculosis-causing bacteria. The problem, however, was that the bacteria fought back, quickly developing resistance. And the rate of decline for new tuberculosis cases has begun to slow during the past decade, with more than nine million people across the globe currently afflicted.
Rifamycins kill their prey by binding to RNA polymerase, the enzyme that kicks off gene expression by transcribing DNA to messenger RNA. However, the exact mechanism by which rifamycins interfere with the process had long remained unknown. A breakthrough came in 2001, when Elizabeth Campbell, a research associate in Darst’s Laboratory of Molecular Biophysics, and her colleagues showed that rifamycins bind next to RNA polymerase’s active center such that the rifamycin acts like a wall, physically blocking RNA from elongating. These results supported a steric-occlusion model for rifamycin action that explained — and continues to explain — past findings.But the newer model, proposed three years ago, describes a very different mechanism. Called the allosteric model, it proposes that rifamycins do, indeed, bind to the enzyme next to the enzyme’s active center, but instead of blocking the elongating RNA molecule, rifamycins transmit a signal to the enzyme’s active center, decreasing a magnesium ion’s ability to bind. Without the magnesium ion, Mg2+, RNA cannot be transcribed.“It was a beautiful model, but there were parts of it that didn’t add up and those parts directly conflicted with the model published in 2001,” says lead researcher Andrey Feklistov, a postdoc in the Darst lab who conducted the research along with several colleagues at Rockefeller, the Waksman Institute of Microbiology at Rutger’s University and The Public Health Research Institute of New Jersey Medical School.The steric-occlusion model suggested that the stronger the rifamycin binds — that is, the sturdier the wall — the better it would work to halt transcription. But according to the allosteric model proposed by a team from The Ohio State University, that wasn’t necessarily the case. Even if rifamycins bind strongly, the enzyme could still be rifamycin-resistant due to a blip along the long signaling pathway. “So we did what scientists do,” says Feklistov. “We took another look.”By testing the same two mutant strains of RNA polymerase that the Ohio team used, ones that had mutations along the proposed signaling pathway, the Darst team found that the mutants were resistant to rifamycin precisely because the antibiotic could not bind tightly to the enzyme. “This suggests that the steric-occlusion model best explains the available biochemical and structural evidence that has been published,” says Feklistov. Moreover, the Darst team found that rifamycins have no effect on metal ion binding to the active center, in direct contradiction to the allosteric model.Understanding the mechanism by which rifamycins kill bacteria allows scientists to better understand how the tuberculosis-causing bacteria develop resistance to the antibiotics — and develop drugs to combat this effect. “At this stage,” says Feklistov, “any evidence, positive or negative, will help focus our attention toward this goal.”
TUBERCULOSIS IN CHILDREN
http://www.medindia.net/news/Tuberculosis-Control-Efforts-Must-Include-Kids-40822-1.htm
William Burman from the Denver Public Health and the University of Colorado Health Sciences Center, Denver, USA, and colleagues said that kids are an often ignored but important part of tuberculosis control efforts. In high-burden settings, children make up as much as 20 percent of new cases of active tuberculosis. Young children are also at high risk of having severe, rapidly progressive forms of tuberculosis. However, nearly 40 years after the development of short-course treatments in adults, there are still major uncertainties about dosing for children of common TB drugs. "Only in recent years has there been a substantial effort to manufacture child-friendly formulations of first-line tuberculosis drugs (such as crushable mini-pills, granules, oral suspensions)," the researchers said. "And in the past 15 years, children have been included in only one study of new agents for tuberculosis: a large Phase 3 trial evaluating once-weekly rifapentine + isoniazid for treatment of latent tuberculosis," they added. The researchers said that including children in drug development is especially critical, as the two main threats to tuberculosis control, HIV-related immunodeficiency and drug-resistant tuberculosis, challenge our ability to develop effective drug regimens. Burman and colleagues outline several traditional barriers to the involvement of children in tuberculosis drug development such as difficulty confirming TB diagnosis, concern about side effects, and regulatory requirements.
William Burman from the Denver Public Health and the University of Colorado Health Sciences Center, Denver, USA, and colleagues said that kids are an often ignored but important part of tuberculosis control efforts. In high-burden settings, children make up as much as 20 percent of new cases of active tuberculosis. Young children are also at high risk of having severe, rapidly progressive forms of tuberculosis. However, nearly 40 years after the development of short-course treatments in adults, there are still major uncertainties about dosing for children of common TB drugs. "Only in recent years has there been a substantial effort to manufacture child-friendly formulations of first-line tuberculosis drugs (such as crushable mini-pills, granules, oral suspensions)," the researchers said. "And in the past 15 years, children have been included in only one study of new agents for tuberculosis: a large Phase 3 trial evaluating once-weekly rifapentine + isoniazid for treatment of latent tuberculosis," they added. The researchers said that including children in drug development is especially critical, as the two main threats to tuberculosis control, HIV-related immunodeficiency and drug-resistant tuberculosis, challenge our ability to develop effective drug regimens. Burman and colleagues outline several traditional barriers to the involvement of children in tuberculosis drug development such as difficulty confirming TB diagnosis, concern about side effects, and regulatory requirements.
Subscribe to:
Posts (Atom)